Simcyp™ Services
Simcyp provides a comprehensive suite of PBPK modeling tools through its p platforms: theSimcyp™ PBPK Simulator, Simcyp™ Biopharmaceutics Toolkit ,Simcyp™ In Vitro Data Analysis Toolkit (SIVA), and Simcyp™ Secondary Intelligence.

These platforms work together to create a unified modeling framework that spans the entire drug development process, from early discovery phases through post-market activities.
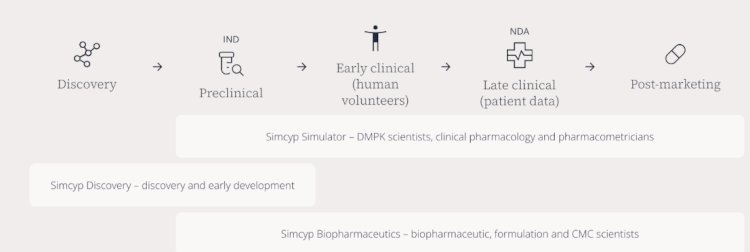
The Simcyp PBPK Simulator predicts drug behavior within the human body, aiding in various stages of drug development. This includes determining optimal dosing for first-in-human trials, optimizing clinical study designs, evaluating new drug formulations, predicting drug-drug interactions, and conducting virtual bioequivalence analyses.
-
Extensive libraries on demographics, developmental physiology, and drug elimination pathways.
-
Advanced mechanistic organ models and compound files.
-
Integrates in vitro data with in vivo outcomes to support informed decision making.
-
Virtual population simulations reflect diverse demographics, ethnicities, and disease states.
-
Includes 29 population libraries and over 100 small and large molecule models.
-
Availability to support compound and population verification documents for regulatory submissions.
Key Features of Simcyp™
Simcyp™ consists of a powerful suite of specialized tools, each designed to address critical phases of drug development with scientific rigor and regulatory alignment.
-
Simcyp™ PBPK Simulator
This flagship module allows for whole-body PBPK modeling that simulates how drugs behave under various physiological and pathological conditions. It provides advanced capabilities for predicting drug-drug interactions (DDIs), enzyme induction/inhibition, and population variability. By virtually modeling different patient groups, the simulator helps researchers make informed decisions about dose selection and trial design—often eliminating the need for exploratory clinical studies. Its outputs are widely accepted by global regulators, making it a key asset in regulatory submissions.
-
Simcyp™ Biopharmaceutics Toolkit
This module focuses on the biopharmaceutical aspects of drug development, enabling prediction of in vivo drug performance based on in vitro data. It models the impact of food, gastrointestinal pH, and physiology on oral drug absorption, helping to assess food effects and formulate products with optimized bioavailability. The toolkit also supports in vitro-in vivo correlation (IVIVC), guiding formulation scientists toward faster, more effective product development and reduced bioequivalence study failures.
-
Simcyp™ In Vitro Data Analysis Toolkit (SIVA)
SIVA provides a structured, automated environment for analyzing complex in vitro data related to metabolic enzymes and drug transporters. It fits kinetic models to experimental data, enabling reliable in vitro–in vivo extrapolation (IVIVE). SIVA ensures consistency in data interpretation and reduces manual calculation errors, making it ideal for early-phase pharmacokinetic modeling, DDI risk assessments, and translational research from bench to clinic.
-
Simcyp™ Secondary Intelligence
This intelligent platform automates the extraction and analysis of Real World Data (RWD) and scientific literature. It assists researchers in conducting systematic reviews, meta-analyses, and pharmacovigilance assessments by summarizing evidence from a wide range of data sources. By reducing the time and manual effort involved in literature reviews, Secondary Intelligence accelerates decision-making and enhances regulatory readiness with traceable, well-organized documentation.
Advantages of Simcyp™
Together, these tools form a unified Simcyp™ ecosystem that empowers drug developers to make better, faster, and safer decisions. From accurate PBPK simulations to robust data analytics and evidence generation, Simcyp™:
-
Accelerates drug development by enabling in silico trials and scenario testing without the need for early human exposure.
-
Supports global regulatory submissions with scientifically validated and accepted simulation reports.
-
Reduces costs by minimizing unnecessary clinical studies and optimizing resource allocation.
-
Improves patient safety and outcomes through better dose predictions and risk assessments.
-
Saves time in research and review by automating data analysis and literature mining.
The pharmaceutical industry faces unprecedented challenges: increasing development costs, longer timelines, and growing regulatory complexity. The Simcyp™ Services portfolio addresses these challenges head-on by providing scientifically robust, regulatory-accepted tools that enable smarter, faster, and more cost-effective drug development.
From the initial stages of drug discovery through regulatory submission and beyond, Simcyp Services provides the predictive insights that transform how pharmaceutical companies approach development decisions. By reducing reliance on expensive and time-consuming clinical studies while maintaining or even improving the quality of development programs, Simcyp is not just a software platform; it's a strategic advantage in today's competitive pharmaceutical landscape.>
Whether you're developing novel therapeutics, optimizing formulations, assessing safety profiles, or preparing regulatory submissions, the comprehensive Simcyp™ Services portfolio provides the tools, expertise, and confidence you need to succeed in modern drug development.
Ready to transform your drug development approach? Contact AKTHealth team at info@akthealth.com to schedule a demonstration and discover how these industry-leading solutions can accelerate your programs while reducing development risks and costs.